Background
In primary immune thrombocytopenia (ITP), immunoglobulin G (IgG) anti-platelet autoantibodies contribute to platelet clearance and impair platelet production. IgG homeostasis is regulated by the neonatal Fc receptor (FcRn), which binds to IgG in a pH-dependent manner and recycles bound IgG. Efgartigimod (EFG), a human IgG1 Fc fragment, is a natural ligand of FcRn engineered to bind competitively to FcRn with high affinity, preventing recycling of endogenous IgG, thereby reducing IgG levels, including pathogenic IgG autoantibody levels.
Methods
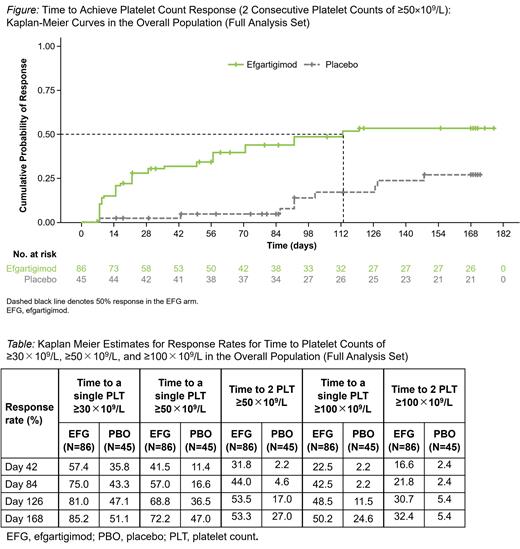
Intravenous (IV) EFG was evaluated in ADVANCE IV, a phase 3, multicenter, randomized, double-blinded, placebo (PBO)-controlled trial (NCT04188379) in adults with persistent or chronic ITP (Broome CM, et al. ASH 2022). Participants who received 2 prior or 1 prior and 1 concurrent ITP therapy with an average of 2 platelet (PLT) counts of <30×10 9/L during screening were randomized 2:1 to receive EFG IV 10 mg/kg or PBO IV for 24 weeks. Concurrent oral corticosteroids, immunosuppressants, dapsone, danazol, fostamatinib, and oral thrombopoietin receptor agonists were permitted but required to remain at the entry dosage and frequency. Participants received weekly dosing of either EFG or PBO (weeks 1-4) followed by response-dependent weekly or every-other-week dosing (weeks 5-16) and then maintained their dosing regimen from weeks 17-24. PLT counts were measured weekly starting at day 7 after treatment initiation. The extent of disease control, defined as cumulative weeks with PLT counts of ≥50×10 9/L in the chronic ITP population, was assessed as a key secondary endpoint. This analysis evaluates trends in time to response in ADVANCE IV. The time to achieve 2 consecutive PLT counts of ≥50×10 9/L was evaluated as a secondary endpoint. Time to 2 consecutive PLT counts of ≥100×10 9/L and time to single counts of ≥50×10 9/L and 100×10 9/L were analyzed as defined a priori. Post hoc Kaplan-Meier (KM) analyses, including time to a single PLT of ≥30×10 9/L and time to the International Working Group (IWG) response, were also performed. Change from baseline in mean PLT counts over time was evaluated based on a mixed model for repeated measurements.
Results
One hundred thirty-one participants (118 chronic ITP, 13 persistent ITP) were randomized (86 EFG, 45 PBO). Least-squares mean estimate changes from baseline in PLT counts were significantly different in EFG group compared with PBO group starting at day 7 (22×10 9/L vs -1×10 9/L; P=0.0293) and remained significant at most time points throughout the study. 38.4% of EFG-treated participants compared with 11.1% of PBO treated participants achieved a PLT count of ≥30×10 9/L in 7 days. The EFG group showed a higher proportion of PLT count responders ≥50×10 9/L on ≥4 occasions until week 12 compared with PBO (34.9% vs 4.4%, respectively). Based on KM analysis, 31.8% of participants receiving EFG and 2.2% of those receiving PBO achieved 2 consecutive PLT counts of ≥50×10 9/L by day 42 (Figure). 41.5% of participants receiving EFG vs 11.4% of those receiving PBO achieved the first PLT count ≥50×10 9/L by day 42. 22.5% of participants receiving EFG vs 2.2% of those receiving PBO achieved the first PLT count ≥100×10 9/L by day 42 (Table). The cumulative number of weeks in disease control was significantly higher in the EFG group compared with PBO (mean [SD]: 6.1 [7.66] vs. 1.5 [3.23]; P=0.0009) (Broome CM, et al. ASH 2022).KM estimates for IWG response by day 42 in the overall (chronic and persistent ITP) population were 29.7% in participants receiving EFG vs 4.6% in those receiving PBO. Overall, EFG was well tolerated; most adverse events were mild or moderate in severity.
Conclusions
In ADVANCE IV, EFG demonstrated a rapid PLT count increase, as early as the first time point measured (7 days after EFG treatment). Irrespective of the specific measure of response utilized, these data demonstrate that at all time points evaluated more participants in the EFG group achieved response compared with the PBO group. In addition to this early response, significantly more sustained responses were observed in the EFG group compared with the PBO group. The response rate, regardless of definition, in participants who received EFG continued to increase over time suggesting that some participants may have delayed responses to EFG. These data further demonstrate early and sustained responses with EFG in ITP.
OffLabel Disclosure:
Broome:Sanofi: Honoraria; Apellis: Honoraria; Alexion: Honoraria; Argenx: Honoraria. McDonald:Amgen: Consultancy; Novartis: Consultancy; Sobi: Consultancy; Grifols: Research Funding; Rigel: Research Funding. Miyakawa:argenx: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Zenyaku Kogyo: Consultancy; Pfizer: Consultancy, Research Funding. Carpenedo:argenx: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Sobi: Honoraria. Kuter:AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui. Immunovant, Incyte, Inmagenebio: Consultancy; AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui, Immunovant, Incyte, Inmagenebio, Ke: Honoraria; Rubius: Current equity holder in publicly-traded company; UpToDate: Patents & Royalties: UpToDate Chapters; Platelet Disorder Support Association: Membership on an entity's Board of Directors or advisory committees; Kezar, Kyowa-Kirin, Merck Sharp & Dohme: Honoraria; Kezar, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Nuvig, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Protagonist, Rigel, Sanofi (Bioveratif), Sanofi (Principia), Sanofi (Genzyme), Sobi (Dova), Takeda, UCB, Up-To-Date, Zafge: Consultancy; Alnylam, BioCryst, Novartis, Rigel, Sanofi (Principia), Takeda (Bioverativ), and UCB: Research Funding. Al-Samkari:Moderna: Consultancy; Pharmacosmos: Consultancy; argenx: Consultancy; Amgen: Research Funding; Novartis: Consultancy; Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding. Bussel:AstraZeneca: Consultancy; Janssen: Consultancy; argenx: Consultancy; Amgen: Consultancy; Novartis: Consultancy; Rigel: Consultancy; Sobi: Consultancy; UCB: Consultancy, Other: Data and safety monitoring board. Hultberg:argenx: Current Employment. Matthijssens:argenx: Current Employment. Ayguasanosa:argenx: Current Employment. De Beuf:argenx: Current Employment. Rodeghiero:Novartis: Consultancy; Amgen: Consultancy; argenx: Consultancy; UCB: Consultancy. Michel:argenx: Honoraria; Sanofi: Consultancy; Sobi: Consultancy; Alexion: Consultancy; Novartis: Consultancy; UCB: Honoraria. Newland:Amgen, Angle, argenx, Dova, Novartis, Ono, Rigel, Shionogi: Consultancy; Amgen, Novartis, and Rigel: Research Funding; Amgen, Angle, argenx, Dova, Novartis, Ono, Rigel, and Shionogi: Honoraria.
Efgartigimod is not currently approved by any regulatory agency for the treatment of primary immune thrombocytopenia (ITP). This is a report of a priori and post hoc analyses of efficacy (time to achieve response) of efgartigimod in primary ITP. Since efgartigimod, a human IgG1 fragment, is a natural ligand of FcRn, it prevents recycling of endogenous IgG antibodies, including anti-platelet autoantibodies in the case of primary ITP.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal